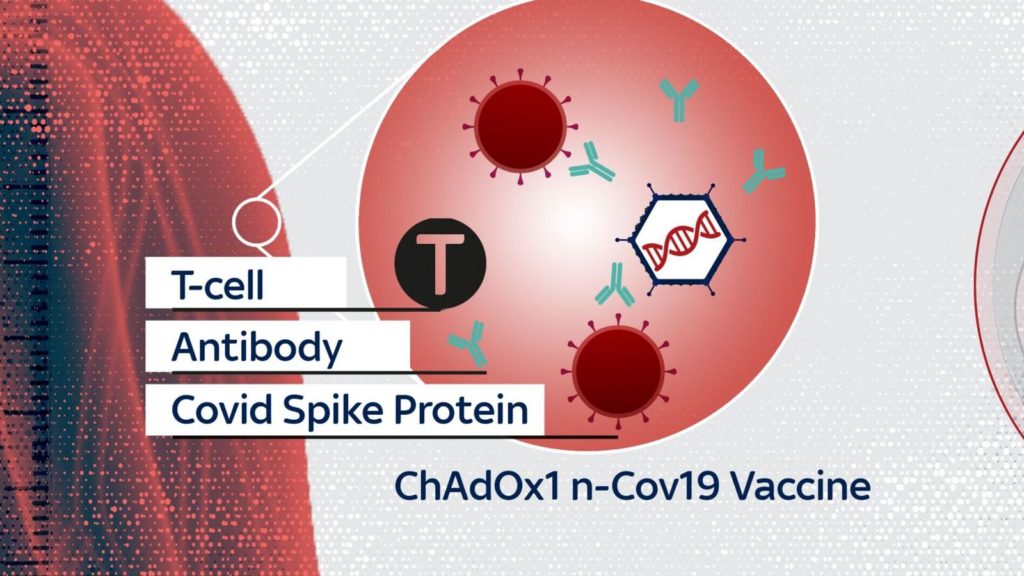
The University of Oxford has announced the development of a vaccine codenamed AZD1222, which has proven to be up to 90% effective in preventing COVID-19, according to tests on thousands of volunteers.
News of the vaccine developed with support from the pharmaceutical giant AstraZenec, comes after Pfizer and Moderna recently announced vaccines which have shown to be 95% and 94.5% effective respectively.
However, the Oxford-AstraZeneca jab is said to be far cheaper, easier to store and get to every corner of the world than the other two. AZD1222 can be stored at normal fridge temperature whiles Pfizer’s vaccine needs to be kept at -70C.
“We have a vaccine for the world,” Professor Andrew Pollard, chief investigator of the Oxford Vaccine Trial at Oxford, said.
According to researchers, when volunteers were given two “high” doses the protection was 62%, but this rose to 90% when people were given a “low” dose followed by a high one. It’s not clear why there is a difference.
Professor Pollard said the researchers “think that by giving smaller first dose we are setting up the immune response better to respond. We will dig in more to that. We have started work this morning.
“It’s critical to understand what everyone is measuring. What counts as COVID disease varies between different protocols.
“If you are only counting hospitalisations then we would have bigger efficacy. We count mild disease and that is much harder to protect against.”
More than 20,000 volunteers were involved, half in the UK, the rest in Brazil.
UK’s Health Secretary, Matt Hancock was pleased with the “really encouraging news of the Oxford-AstraZeneca vaccine that the government had “obviously been backing since the start.
“I’m really very pleased, I really welcome these figures – this data – that show that the vaccine in the right dosage can be up to 90% effective.
“Of course, it’s vital that the independent regulator – the MHRA (Medicines and Healthcare products Regulatory Agency) – will need to look at the data, will need to check to make sure that it’s effective and safe, of course.
“But we’ve got 100 million doses on order and should all that go well, the bulk of the rollout will be in the New Year.
“This homegrown vaccine is easier to administer as well than the Pfizer vaccine because it doesn’t need to be stored at -70C.
“So having two vaccines that appear to have effectiveness, done right, in the 90% range is really really good news. Fantastic news.”
Prime Minister Boris Johnson added in a tweet, “Incredibly exciting news the Oxford vaccine has proved so effective in trials. There are still further safety checks ahead, but these are fantastic results.
“Well done to our brilliant scientists at @UniofOxford & @AstraZeneca, and all who volunteered in the trials.”
The UK has pre-ordered 100 million doses, with four million expected to be distributed by the end of the year if the vaccine is approved by the medicines’ regulator.
AstraZeneca will also seek an Emergency Use Listing from the World Health Organisation, which will allow for “accelerated pathway to vaccine availability in low-income countries”.