The European Medicines Agency (EMA) has announced that it has found a “possible link” between the Johnson & Johnson COVID vaccine;and “very rare” cases of unusual blood clots. As a result, the agency admonished that unusual blood clots with low blood platelets, should now be;listed as “very rare” side effects of the vaccine.
The EMA, however, stressed that the “overall benefits of the vaccine in preventing COVID-19 outweigh the risks of side effects”.
The new warning comes after eight “serious” blood clot cases were recorded in the US, with a person dying. The J&J vaccine, which is given;as a single jab, had;been; administered to more than seven million people in the US, as of 13th April.
The EMA noted that all eight people were under the age of 60 and developed;clots within three weeks of vaccination, with the majority of cases being in women. Officials said the cases were “very similar to the cases that occurred with the coronavirus vaccine developed by AstraZeneca”.
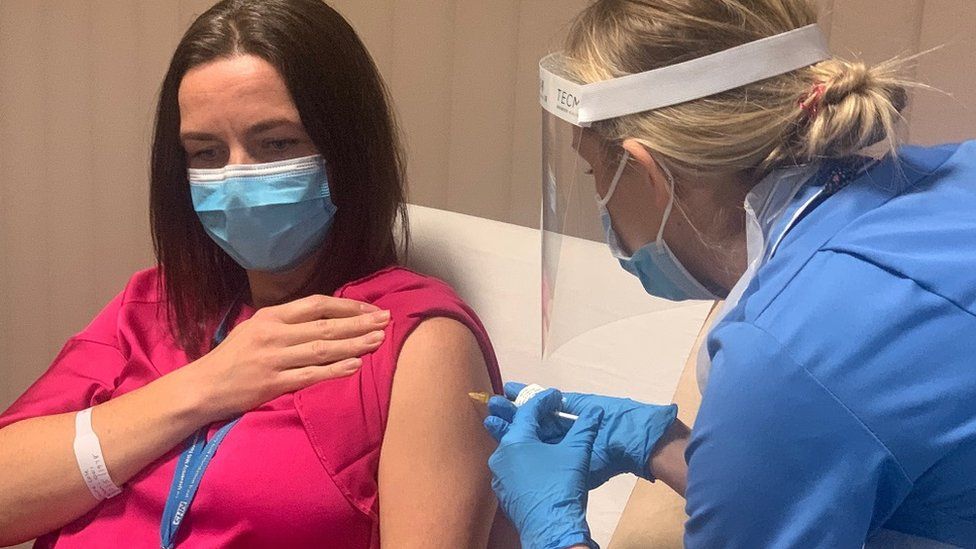
The EMA also intimated that it will “continue to monitor the vaccine’s safety and effectiveness and provide the public with the latest information” after the rollout in Europe was;halted over blood clot concerns.
Although the risk of such serious side effects is “very low”, EMA officials gave Europeans a list of symptoms to watch out for within three weeks of getting a J&J vaccine.
The symptoms to be;reported include Shortness of breath, Chest pain, Leg swelling, Persistent abdominal pain, Severe or persistent headaches, Blurred vision or Tiny blood spots under the skin beyond site of injection.
J&J suspension could shake vaccine confidence
Experts have expressed concern that the temporary suspension of J&J’s shot could further shake vaccine confidence and complicate worldwide COVID-19 immunization efforts.
Last week, J&J halted its European rollout after US officials recommended a pause in the vaccine. The pharmaceutical company advised European governments to store their doses until the EMA issued guidance on its use. Widespread use of the shot in Europe had, however, not started yet.
Countries including Italy, Romania, the Netherlands, Denmark and Croatia have put their J&J doses into storage. South Africa also suspended its use of the vaccine in the wake of the US suspension.
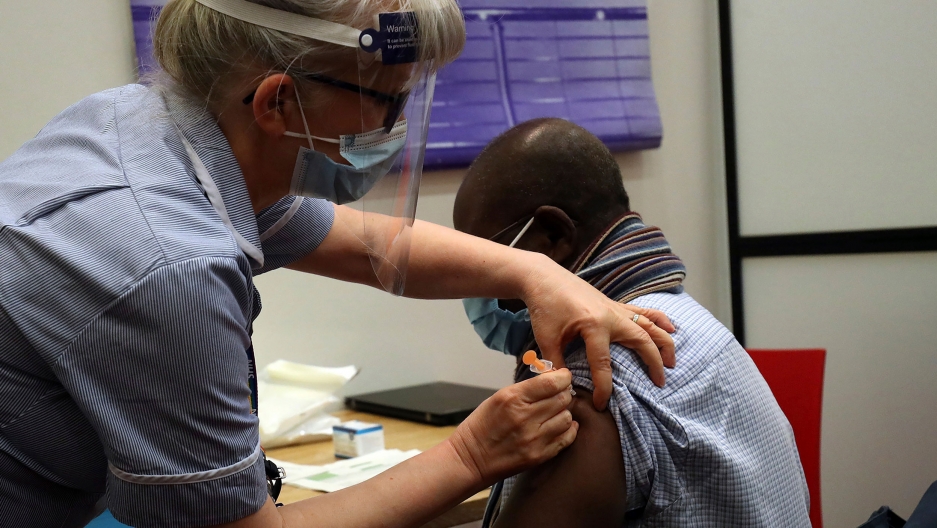
Health officials noted that the blood clots linked to the J&J vaccine are occurring in unusual parts of the body, such as veins that drain blood from the brain. The affected patients also have abnormally low levels of blood platelets, a condition normally linked to bleeding, not clotting.
Both the J&J and AstraZeneca vaccines, as well as the Sputnik V vaccine are made with the same technology. They train the immune system to recognize the spike protein that coats the coronavirus. To do that, they use a cold virus, called an adenovirus, to carry the spike gene into the body.
Eleanor Riley, a professor of immunology and infectious diseases at the University of Edinburgh has noted that the mechanism used to produce both AstraZeneca and J&J may be “triggering” the clots.
“Suspicion is rising that these rare cases may be triggered by the adenovirus component of the AstraZeneca and J&J vaccines.
“While more data was needed, it remains the case that for the vast majority of adults in Europe and the USA, the risks associated with contracting COVID-19 far, far outweigh any risk of being vaccinated.”
Read More: Chad President, Idriss Deby dies ‘in clash with rebels’




















